Case Reports
NUHS policy specifies that all research projects involving human subjects must be reviewed by the Research Committee and approved by the Institutional Review Board (IRB); however, a case report (1 patient) or case series (more than 1 patient) that report the observations of a subject(s) receiving the normal standard of care is generally not considered research because there is no intent to test a hypothesis via systematic analysis. Use the Decision Tree (below) and the NUHS Guidance for Case Studies to assist you in determining if your case report/case series is research.
Research or Non-Research Decision Tree for IRB Submission
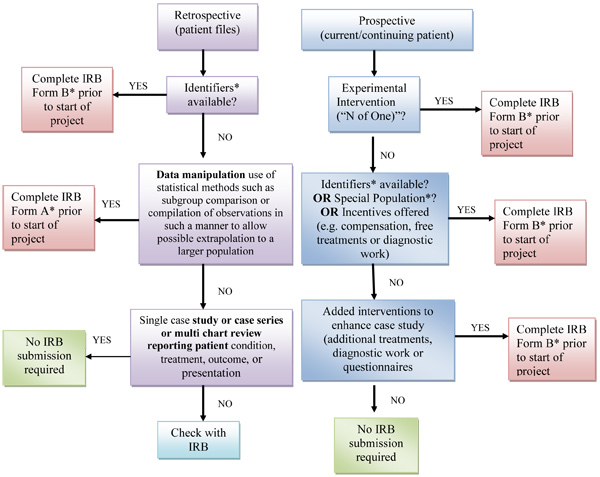
* See NUHS Guidance Case Studies for more information.


